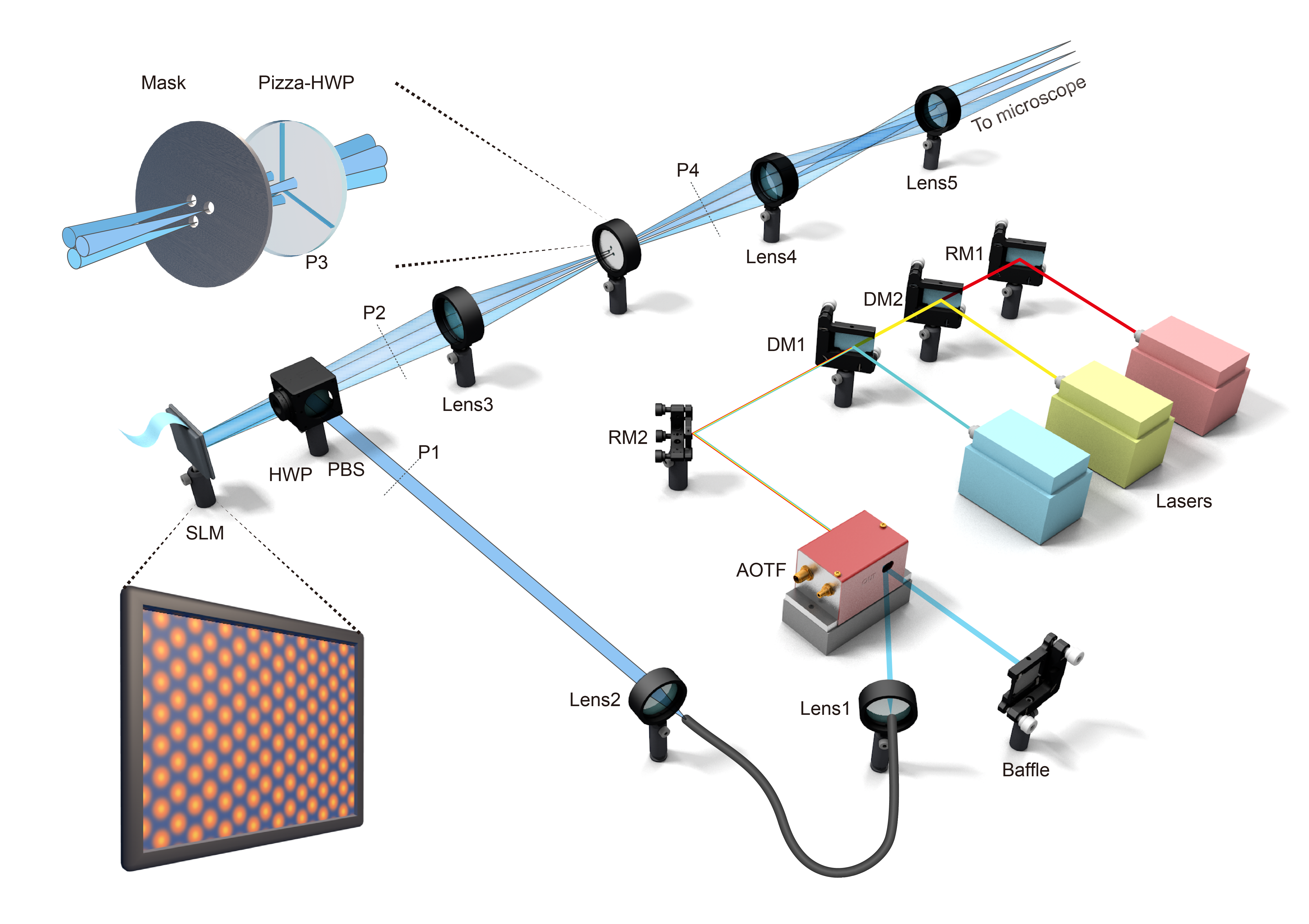
In nature, the triangle is the simplest and most stable 2D structure, while the hexagon represents the optimal spatial arrangement. However, in the field of optics, conventional structured illumination super-resolution microscopy (SIM) has long relied on the complex assembly of "hexagonal patterns" through the superposition of three "striped patterns—achieved by rotating one-dimensional stripe illumination at three angles. Drawing inspiration from simplicity and efficiency of triangular structures, Professor Peng Xi’s team in Peking University developed a Triangular-beam Interference Structured Illumination Microscope (3I-SIM), pushing the spatiotemporal boundaries of live-cell observation with its 'triangular architecture'. The findings, titled "Triangle-beam interference structured illumination microscopy," was recently published in the high-impact journal Nature Photonics, offering a powerful new tool for dynamic life science research.

Subcellular structures exhibit intricate dynamics across spatial and temporal scales, forming the foundation of biological activity. Deciphering their movement trajectories and interactions is key to understanding cellular functions and regulatory processes. Structured illumination super-resolution (SR) microscopy has gained prominence in live-cell studies due to its capability to achieve high resolution in both space and time simultaneously.
In the Taoist system, “Tao begets one, one begets two, two begets three, and three begets all things." Conventional two-dimensional structured illumination microscopy (2D-SIM) surpasses the optical diffraction limit, but its working principle analogous to piecing together a puzzle step by step: it generates one-dimensional stripes patterns via Young’s double-slit interference to achieve 1D resolution enhancement. For 2D resolution improvement, the stripes must be rotated by ±60°, thereby obtaining super-resolution information in three orientations. In contrast, 3I-SIM innovatively employs triangular-beam interference to achieve 2D lattice modulation, expanding high-frequency spatial information in both dimensions in a single exposure. With only 7 raw frames needed for reconstruction, 3I-SIM significantly effectively mitigates photobleaching during acquisition. The unidirectional phase-shift characteristicof the 2D lattice modulation eliminates the multi-angle rotation required in traditional 2D-SIM, minimizing redundant spectral sampling. Additionally, this desig neliminates the requirement for pattern orientation matching in rolling reconstruction, freeing the system from dependence on same-orientation stripe patterns. As a result, 3I-SIM achieves an imaging speed of up to 1,697 Hz under single-frame rolling reconstruction.
Furthermore, the research team systematically analyzed the impact of polarization strategies on modulation capability. Breaking the framework constraints of conventional azimuthal polarization, they proposed a radial polarization strategy, elevatingthe high-frequency modulation capability to a level comparable to that of conventional 2D-SIM. Combined with their in-housedeveloped, highly robust physical reconstruction algorithm, this innovation significantly improves the system’s noise resistance.
Compared to conventional 2D-SIM, 3I-SIM effectively reduces phototoxicity and photobleaching in dynamic imaging while achieving a frame rate of up to 1,697 Hz. The research team also developed 3I-Net, a deep learning-based reconstruction method employing a co-supervised deep learning approach, which enables ultrahigh detection sensitivity under photon-limited conditions and substantially improves reconstruction quality. This breakthrough overcomes the limitations of conventional imaging in low signal-to-noise scenarios, allowing for 13-hour, 100,000-frame super-resolution imaging of light-sensitive structures such asneuronal growth cones and high-speed SR capture of transient weak signals such as endoplasmic reticulum-associated actin.

Figure 1 | Principle and performance characterization of 3I-SIM. a. Schematic of the 3I-SIM imaging system. b. Comparison of conventional two-beam interference modulation and 3I-SIM triangular-beam interference modulation patterns. c. Dual-color imaging of synaptonemal complexes, comparing widefield and super-resolution results. d. Imaging of Nile Red–labeled COS-7 cells, comparing widefield and super-resolution results.
3I-SIM excels in gentle, high-speed organelle imaging in live cells. The research team demonstrated stable long-term imaging of actin filaments in HUVEC , acquiringover 6,000 frames at 1 Hz. In high-speed mode, 3I-SIM achieved a rolling reconstruction frame rate of up to 1697 Hz,, enabling the capture of transient fluctuations during endoplasmic reticulum ring closure. Leveraging its ultrahigh spatiotemporal resolution, 3I-SIM also enables high-quality multicolor imaging, revealing intricateinteractions between the endoplasmic reticulum, late endosomes/lysosomes, and microtubules during contact, remodeling, and dynamic regulation.
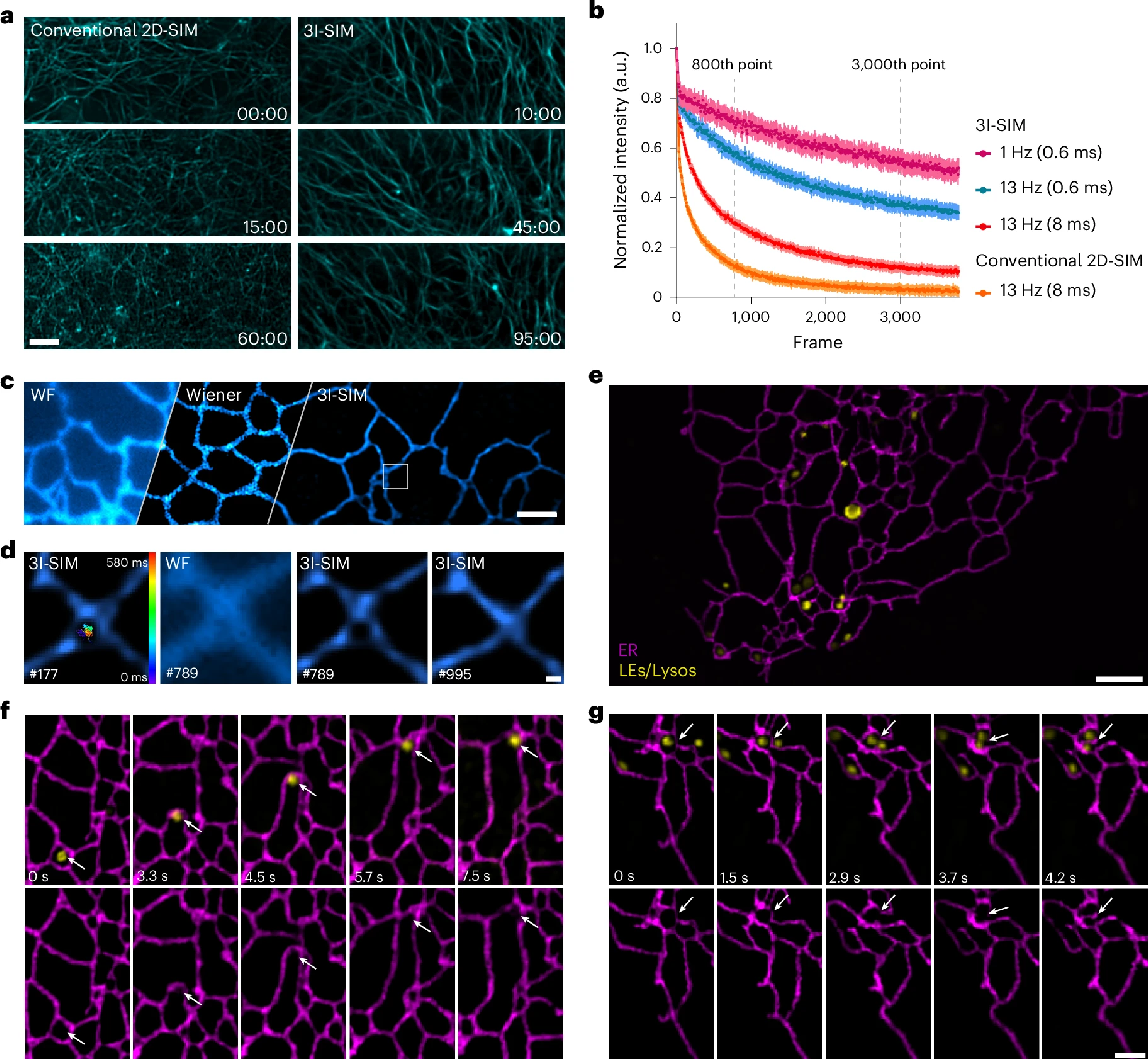
Figure 2 | 3I-SIM enables faster and gentler live-cell organelle imaging. a, b. Comparison of photobleaching on fluorescent proteins under different illumination modes and excitation strategies between conventional 2D-SIM and 3I-SIM. c, d. Dynamic ER imaging at 1,697 Hz, with magnified details and trajectories of ER ring closure events. e–g. Interactions between LEs/Lysos and ER, including local deformations during rapid movement and coordinated ER tubule remodeling during "kiss-and-run" events.
Through hardware-software co-optimization, 3I-SIM delivers gentler and faster super-resolution imaging, representing a significant breakthrough in live-cell observation. It demonstrates exceptional capability in resolving subcellular dynamics at the 100-nanometer scale, providing robust support for life science research.The technology has been made openly available with the team research releasing a comprehensive resource package covering hardware design, software control, reconstruction algorithms, and deep learning models, along with accompanying datasets. The 3I-SIM system can be flexibly upgraded on common 2D-SIM platforms, effectively lowering the technical barrier and enabling more research teams to access next-generation live-cell super-resolution imaging.
Professor Peng Xi (College of Future Technology, Peking University) and Dr. Meiqi Li (School of Life Sciences, Peking University) are co-corresponding authors. PhD candidates Yunzhe Fu and Yiwei Hou (College of Future Technology) are co-first authors. Key contributions were also made by Ph.D. student Qianxi Liang, postdoctoral researchers Xin Chen and Boya Jin from Peng Xi’s group. The work received support from Professor Chen Zhixing’s team (College of Future Technology), Professor Pengli Zheng’s team (School of Life Sciences), and Beijing Airy Technologies Co., Ltd. This research was supported by funding from the National Key Research and Development Program of China and the National Natural Science Foundation of China.
Link: https://www.nature.com/articles/s41566-025-01730-0