The 2014 Nobel Prize in Chemistry was awarded to super-resolution fluorescence microscopy, which exploits the chemical switching properties of fluorescent molecules (PALM/FPALM/STORM) or stimulated emission depletion (STED) to achieve super-resolution imaging beyond the diffraction limit. However, two significant bottlenecks prevent them from being used in live cells.
(1) the phototoxicity of super-resolution (SR) limits the observation time of intricate physiological processes in living cells.
(2) The temporal and spatial resolution cannot be simultaneously improved, limited by the number of photons emitted per unit time of fluorescent molecules.
To achieve the spatial resolution limit of living cells, existing super-resolution imaging tools require strong illumination power (kW~MW/mm2), unique fluorescent probes, and long exposure times (> 2 s). The intense bleaching caused by strong illumination power can destroy the integrity of fluorescence structures, and long exposure times lead to motion artifacts during image reconstruction and reduce the effective resolution. The main determinant of the spatial resolution of live-cell super-resolution (SR) microscopes is the maximum photon flux that can be collected. Thus improvements based on optical hardware or fluorescent probes fail to push the spatiotemporal limit of live-cell SR imaging to 60 nm with millisecond temporal resolution.
On November 16, 2021, Prof. Liangyi Chen's team at the School of Future Technology, Peking University, collaborated with Prof. Haoyu Li's team at Harbin Institute of Technology to achieve this goal and published "Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy" in Nature Biotechnology .
To further increase the effective resolution for a given photon flux, they take advantage of a priori knowledge about the sparsity and continuity of biological structures to develop a two-step iterative deconvolution algorithm that increases the resolution of SR microscopes nearly twofold. The method, sparse structured illumination microscopy (Sparse-SIM), achieves ~60-nm resolution at a frame rate of up to 564 Hz, allowing it to resolve intricate structures, including small vesicular fusion pores, ring-shaped nuclear pores formed by nucleoporins, and relative movements of inner and outer mitochondrial membranes in live cells. Sparse deconvolution can also be used to increase the three-dimensional resolution of spinning-disc confocal-based SIM, even at low signal-to-noise ratios, which allows four-color, three-dimensional live-cell SR imaging at ~90-nm resolution. Overall, sparse deconvolution will be useful to increase the spatiotemporal resolution of live-cell fluorescence microscopy.
1 | Application example: Sparse-SIM ultrafast live-cell imaging reveals nuclear pore structures and early fusion pore of insulin vesicles
In live-cell imaging, Sparse-SIM can resolve ring-shaped nuclear pore structures labeled with different nuclear pore proteins (Nup35, Nup93, Nup98, or Nup107) that are similar in shape size to 100 nm fluorescent beads under the conventional 2D-SIM (Figure 1c, 2d). Since the camera pixel size is similar in size to the aperture diameter, the measured fitted diameter of the nuclear pores is comparable to the resolution of Sparse-SIM. The calibrated Nup35 and Nup107 pore diameters were ~66 ± 3 nm and ~97 ± 5 nm, respectively, while the Nup98 and Nup93 diameter sizes were in this range (Figure 1e, 1f), and the results were consistent with diameters previously obtained in fixed cells with other super-resolution imaging methods. Interestingly, 12 min SR imaging showed changes in nuclear pore shapes in a live COS-7 cell, possibly reflecting dynamic reorientation of individual nuclear pore complexes on the nuclear membrane to or away from the focal plane (Figure 1g), which is difficult to observe with other super-resolution methods.
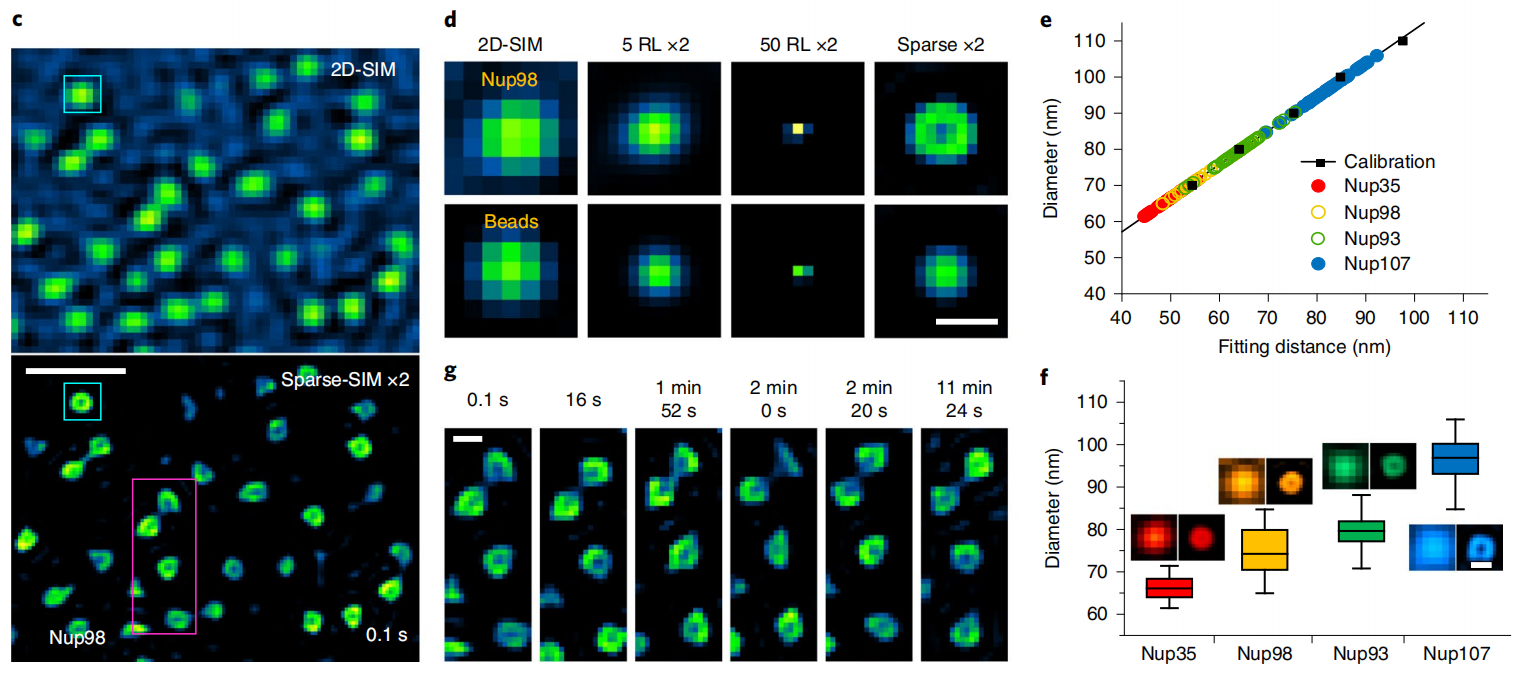
Figure 1 | Sparse-SIM resolves dynamic processes of nuclear pore proteins. (c) A typical example of dynamic ring-like nuclear pores labeled with Nup98-GFP in live COS-7 cells was observed with Sparse-SIM for more than 10 min. The upper and lower regions show the images under 2D-SIM and Sparse-SIM. (d) Comparison of the snapshot of the nuclear pore structure in the cyan box in (c) with the results of 100 nm fluorescent beads under different reconstruction methods (2D-SIM, after 20 times RL deconvolution, after 50 times RL deconvolution, Sparse-SIM). (e) Actual diameters of Nup35-GFP (red), Nup98-GFP (yellow), Nup93-GFP (green), and Nup107-GFP (cyan) labeled nuclear pore structures obtained after correction ((see article for details). (f) Average diameter rings of Nup35 (66 ± 3 nm, n = 30), Nup98 (75 ± 6 nm, n = 40), Nup93 (79 ± 4 nm, n = 40), and Nup107 (97 ± 5 nm ,n = 40). The left and right montages are after conventional Wiener reconstruction or sparse deconvolution, respectively. (g) The magenta boxes in (c) are enlarged and shown at 6 time points. Scale bars: (c) 500 nm; (d, g, f) 100 nm.
By rolling reconstruction, Sparse-SIM achieves a temporal resolution of up to 564 Hz, which enables identification of VAMP2-pHluorin-labeled, smaller fusion pores of insulin vesicle in INS-1 cells (e.g. ~61 nm pore size, Figure 2o,2p). They appear early in vesicle fusion, have a small pore size (mean diameter ~87 nm) and short duration (9.5 ms), and cannot be identified by the previously conventional TIRF-SIM. On the other hand, the identified stable fusion pore appears late in vesicle fusion with a large pore size (average diameter ~116 nm) and long duration (47 ms) (Figure 2q), a previously seen structure [2]. The early fusion pore states of the vesicles found here are difficult to verify directly by other SR imaging tools. However, their frequency of occurrence is consistent with the report that "small fusion pores are much less likely to occur than large fusion pores," which was observed more than 30 years ago using fast freeze-etching electron microscopy.

Fig. 2 | Sparse-SIM analysis of ultrafast insulin metabolism process. (n) Average diameters of different types of vesicles. (o) Representative montage images of vesicle fusion events. (p) Kymograph maps of vesicle secretion were recorded by TIRF-SIM (top) and Sparse-SIM (bottom). (q) Average opening time (left), diameter (middle), and duration (right) of early (yellow) and stable (green) fusion pores. Scale bars: (a, e, m (top)) 5 μm; (b, j) 500 nm; (h, m (bottom)) 1 μm; (k) 100 nm.
2 | Application example: sparse deconvolution confers a method to improve the resolution of fluorescence microscopy in general
Unlike the popular deep-learning enabled SR reconstruction, the two a priori knowledge we used here are universal to fluorescence microscopy, which depends neither on the sample nor the specific modality of the microscope. Therefore, sparse deconvolution is a general fluorescence microscopy computational SR algorithm that can be widely used to enhance the resolution of other fluorescence microscopy modalities and to observe the fine structure and dynamics of different cellular substructures.
For example, sparse deconvolution can improve the resolution of a commercial spinning-disc confocal structured illumination microscope (SD-SIM) to 90 nm in the XY direction and 250 nm in the Z direction. Therefore, the outer membrane of all mitochondria in a cell of 7 μm in depth can be clearly observed (Figure 3). Similarly, if sparse deconvolution is combined with commercial SD-SIM, three-dimensional, four-color super-resolution imaging on living cells can be easily achieved. Sparse deconvolution can also be combined with an expansion microscope, a wide-field, point-scan confocal, STED, and miniaturized two-photon microscope. All come with a nearly two-fold increase in their spatial resolutions. Thus, the proposed sparse deconvolution will help biomedical researchers resolve the intricate dynamic structures in live cells using a wide variety of fluorescence microscopes.

Figure 3 | Sparse SD-SIM resolve the 3D mitochondrial outer membrane network in a live cell. (k) Three-dimensional distribution of mitochondrial outer membranes (labeled with Tom20-mCherry) of living COS-7 cells, with the color is representing depth. (l) SD-SIM raw data before the deconvolution were shown horizontally (left) and vertically (right) in the upper panel, while images after sparse deconvolution were shown at the bottom. Scale bars: (k) 5 μm; (l) 1 μm.
In summary, sparse deconvolution enables computational fluorescence SR imaging, different from current SR methods based on specific physical principles or unique fluorescent probes. Sparse-SIM achieves the highest spatial (60 nm), temporal resolutions (564 frames/sec), and longest imaging time (more than 1 hour) for live-cell SR imaging up till now. By integrating it with most fluorescence microscopes, sparse deconvolution can also improve spatial resolutions. The paper was highlighted in Faculty Opinions as "Technique breakthrough"(10.3410/f.741163644.793590090) and selected as "2021 Light10 in China."

(https://new.qq.com/omn/20211230/20211230A04LGH00.html)
(promotional video)
Weisong Zhao, a Ph.D. student at Harbin Institute of Technology, Shiqun Zhao, and Liuju Li, postdocs at Peking University, are co-first authors. Prof. Haoyu Li of the School of Instrument Science and Engineering at Harbin Institute of Technology and Prof. Liangyi Chen of the School of Future Technology at Peking University are the paper's co-corresponding authors. This work was funded by the National Natural Science Foundation of China and other funding sources.

