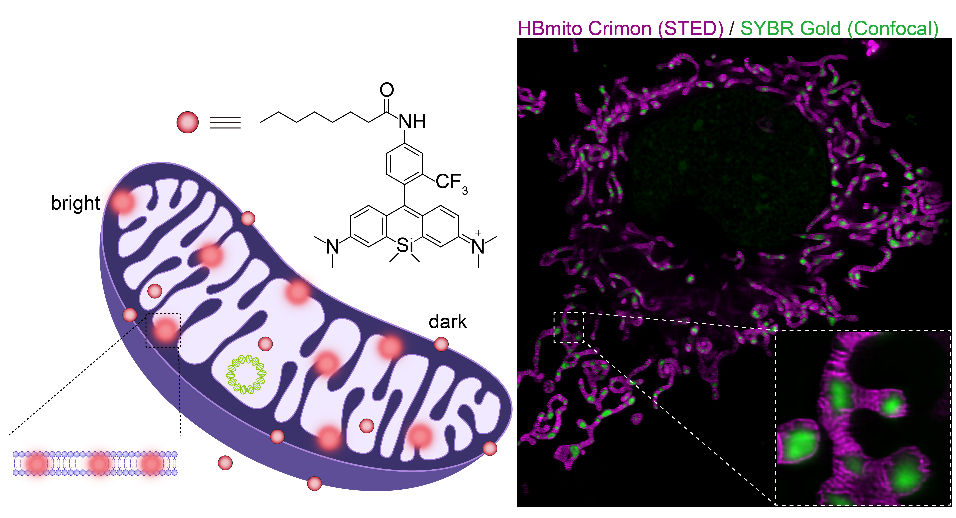
Abstract
Beijing, May 27, 2024 - In the microscopic world of cells, mitochondria function like sophisticated power plants, regulating chemical reactions on the inner mitochondrial membrane (cristae) to provide the essential energy for life activities. These tiny organelles are crucial not only for energy conversion but also for cell growth, stability, signal transduction, and even apoptosis. The unique properties of mitochondria stem from their exclusive genetic information encapsulated by the inner mitochondrial membrane - mitochondrial DNA (mtDNA). Scientists are eager to further explore the distribution patterns of mtDNA within the mitochondrial network and the interaction between mtDNA and mitochondrial cristae. Research team led by Prof. Peng Xi inPeking University and Hebei University has developed a novel fluorescent probe, HBmito Crimson, which unveils the mysterious interaction between mitochondrial cristae and their internal DNA (mtDNA). This work has been published in Light: Science and Applications.
Inside cells, mitochondria play a crucial role as the “powerhouses” of the cell. It isnot only responsible for energy production, but also involved in key signaling pathways that regulate cell proliferation, homeostasis, and apoptosis. The cristae, as part of the inner membrane, have a fine and complex structure and is essential for maintaining mitochondrial function. Mitochondria possess their own genetic code, akin to an organ within the body but with its independent DNA, adding to their mysterious origins and functions. Mitochondrial DNA (mtDNA) is located in the mitochondrial matrix, containing 37 genes encoding 14 mitochondrial proteins, playing a crucial role in ATP production. mtDNA is packaged by proteins to form nucleoid, and the movement of nucleoids is restricted within the mitochondria due to the cristae acting as barriers. Mitochondrial fusion and fission involved in cristae remodeling are essential for the distribution and maintenance of mtDNA. However, the dynamic characteristics of cristae arrangement facilitating mtDNA distribution within the entire mitochondrial network remain unexplored.
Mitochondria typically have a diameter of about 500 nm, with cristae spacing generally less than 70 nm. Traditional microscopy techniques cannot observe these dynamic processes. The development of super-resolution microscopy, particularly stimulated emission depletion (STED) microscopy, has made visualizing mitochondrial cristae dynamics possible, offering spatial and temporal resolutions of ~ 50 nm and 1 frame per second, respectively. However, STED requires high-intensity depletion beams to enhance resolution, necessitating dyes with high brightness and high stimulated emission cross-sections (i.e., low saturation power) when specifically targeting the inner mitochondrial membrane. Traditional dyes can only sustain a few frames under STED, and newly developed dyes do not meet the requirements for dynamic imaging over extended periods.
In this research, the joint team from Peking University and Hebei University developed a novel fluorescent probe named HBmito Crimson ("HB" has a double meaning, representing both "High Brightness" and "HeBei", where it is developed.). This probe features high brightness, excellent photostability, lipid membrane fluorogenicity, and low STED saturation power, thus highly suitable for long-term STED super-resolution microscopy of mitochondria dynamics in live cell. Withthis probe, the research team successfully achieved continuous low-power imaging of over 500 frames, visualizing inner membrane dynamics with a spatial resolution of 40 nm (Fig. 1).
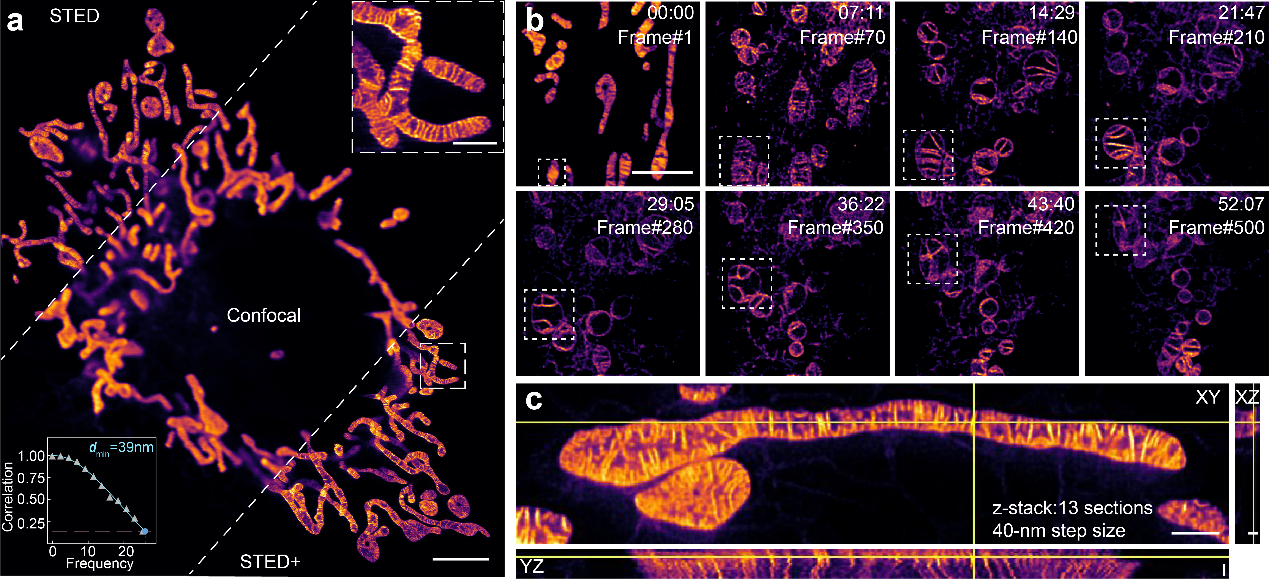
Figure 1 Multi-dimensional imaging of mitochondrial inner membrane stained with HBmitoCrimson. (a) Comparison of confocal, STED, and STED deconvolution results. (b) A long period time-lapse STED imaging of mitochondria, with 500 frames. (c) 3D-STED imaging of mitochondria.
Using HBmito Crimson and SYBR Gold for dual-color STED/confocal imaging of the inner membrane and mtDNA, it was found that mtDNA tends to habitat at mitochondrial tips or branch points, exhibiting an overall spatially even distribution. Differences in mtDNA distribution between small individual mitochondria and interconnected mitochondrial networks suggest that mitochondria of different sizes may serve distinct functions. During mitochondrial dynamics (Fig. 2), it was observed that mitochondrial networks form through fusion events and the generation of new branches. The authors first visualized the cristae dynamics and the mtDNA changes in distribution when mitochondria extend the branch. Interestingly, the authors found that fusion events tend to occur near mtDNA, possibly due to the void matrixes between cristae clusters,where mtDNA is located, resulting in less pressure during cristae remodeling. This close association likely also allows for the efficient transmission of mtDNA between different mitochondria during fusion. Additionally, observations of mitochondrial fission revealed spatial links between mtDNA replication and fission sites, enabling proper genetic material segregation and even distribution throughout the mitochondrial network.
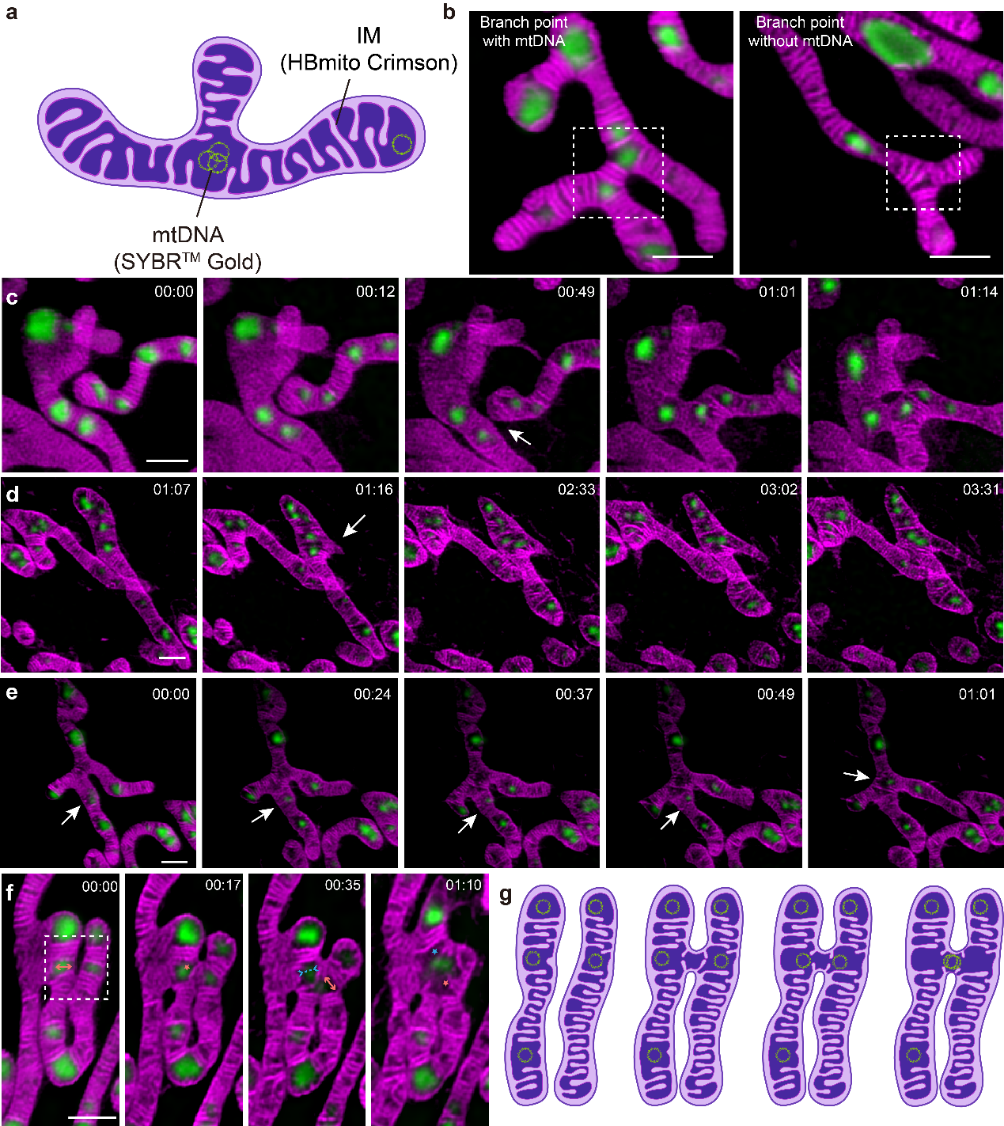
Figure 2 Mitochondrial dynamics associated with mtDNA. (a) Cartoon showing that mtDNA prefers to be distributed at the tips and branch points. (b) Presence and absence of mtDNA at branch points. (c) Branch point formation by mitochondrial fusion. (d) Branch point formation by emersion of a new branch. (e) The mtDNA on the mitochondrial branch moves to the branch point. (f, g) Cristae dynamics during mitochondrial fusion.
mtDNA plays an important role in maintaining cristae structure. Conversely, maintenance of mtDNA distribution also requires normal cristae dynamics. In cases of apoptosis and ferroptosis, cristae structure is disrupted, leading to mtDNA distribution disorder. The authors observed the dynamic processes of inner membrane herniation and mtDNA leakage during late apoptosis, as well as the shrinkage and fragmentation of mitochondria along with a convergence of mtDNA in ferroptosis, reflecting significant morphological changes and mitochondrial damage associatedwith this mode of cell death.
In summary, this research reveals the intricate relationship between mitochondrial membrane dynamics and mtDNA distribution with an ultra-high spatial and temporal resolution of 40nm. The combination of advanced imaging technology and novel mitochondrial inner membrane probes makes low phototoxicity live-cell STED imaging possible and demonstrates the great potential of new technology in next-generation mitochondrial research. Understanding these mechanisms will help elucidate the role of mitochondria in cell physiology, human diseases, and aging.
Leading Authors' Bibliography
Wei Ren, Ph.D.candidate at the College of Future Technology, Peking University, and Xichuan Ge, master student at the College of Chemistry and Material Science, Hebei University, are co-first authors. Prof. Peng Xi from the College of Future Technology, Peking University, Prof. Baoxiang Gao from the College of Chemistry and Material Science, Hebei University, and Senior Engineer Chunyan Shan from the School of Life Sciences, Peking University, are co-corresponding authors.
This work was supported by the National Key R&D Program of China, and National Natural Science Foundation of China, and received assistance from the National Center for Protein Sciences at Peking University in Beijing, China, Abberior China, and Optofem Technology Limited. The HBmito Crimson probe product is commercially available fromMedChemExpress (MCE,https://www.medchemexpress.cn/hb-imm-d-red.html).

Wei Ren is a Ph.D. candidate at the College of Future Technology, Peking University, supervised by Prof. Peng Xi. His primary research areas include super-resolution microscopy technologies, specifically stimulated emission depletion microscopy (STED) and image scanning microscopy (ISM), as well as mitochondrial dynamics imaging.His research findings have been published in journals such as Light: Science & Applications and eLight. He holds one granted invention patent and has delivered oral presentations at international conferences like FOM and CIOP. Wei Ren has been honored with the Peking University President Scholarship among other accolades.

Xichuan Ge earned his master's degree in 2023 from the College of Chemistry and Material Science, Hebei University, under the guidance of Prof. Baoxiang Gao. His research mainly focuses on mitochondrial super-resolution imaging, the design and synthesis of fluorescent probes, and the biological applications of super-resolution imaging. His work has been published in journals such as Light: Science & Applications and Chemical Communications.

Chunyan Shan is a senior engineer at the School of Life Sciences, Peking University, and the head of the Optical Imaging Platform at the National Center for Protein Sciences (Peking University) and the School of Life Sciences Public Instrument Center. She focuses on the research and application of optical microscopy imaging technology. She graduated with a bachelor's degree from Beijing Institute of Technology in 2007 and received her Ph.D. in cell biology from Peking University in 2013.Since 2013, she has been primarily engaged in providing platform support services related to imaging technology, responsible for the development and application of novel fluorescence microscopy techniques. These include super-resolution fluorescence imaging, multidimensional fluorescence imaging techniques, live-cell imaging, novel fluorescence labeling techniques, and multiplexed tissue pathology techniques. She has made progress in areas such as live-cell super-resolution imaging, multidimensional fluorescence labeling and imaging techniques, and single-cell spatial genomics. Her related findings have been published in journals such as Nature Communications, Nanophotonics, and Scientific Reports.

Prof. Baoxiang Gao is a professor and doctoral supervisor at the College of Chemistry and Material Science, Hebei University, and a senior member of the Chinese Chemical Society. He graduated with a bachelor's degree from Beijing Institute of Technology in 1998 and received his Ph.D. from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, in 2006. He has been working at Hebei University since 2006. In 2011, he received the Hebei Outstanding Youth Fund, and in 2012, he was selected for the Ministry of Education's New Century Excellent Talents Support Program. From 2015 to 2016, he studied single-molecule fluorescence and super-resolution imaging technology in Prof. Philip Tinnefeld’s lab at the Center for Life Science, Braunschweig University of Technology, Germany. To date, he has published over 40 research papers in well-known academic journals such as Advanced Functional Materials, Analytical Chemistry, and Organic Letters.

Prof. Peng Xi is a Boya Professor at the College of Future Technology, Peking University, and a Fellow of International Association of Advanced Materials(FIAAM).He has received the National Distinguished Young Scholar award and serves as the chief scientist of the National Key R&D Program of China.He is dedicated to researching new super-resolution microscopy technologies. He currently serves as an editorial board member for five international academic journals, including Advanced Photonics. He has published over 90 SCI-indexed journal papers in international journals such as Nature and Nature Methods, with a total impact factor exceeding 700 and more than 6,000 citations. In 2016, he received the China Optics Important Achievement Award, andin 2022, he was awarded the Guangdong Provincial Natural Science Second Prize (second place). He has been granted 3 U.S. patents and 19 Chinese patents and has edited 2 books. He has been invited to give plenary talks at international conferences organized by OPTICA (formerly OSA) and SPIE.
Link to the paper:https://www.nature.com/articles/s41377-024-01463-9
:

