There is a growing demand for non-invasive insights into the complex three-dimensional subcellular dynamics within living tissues on the frontier of biological research. To achieve simultaneous optimization of spatial resolution, imaging depth, and phototoxicity, Professor Peng Xi's group at Peking University has ingeniously combined confocal scanning imaging with structural illumination super-resolution, and have proposed a multi-confocal image scanning microscopy (MC-ISM). By optimizing pinhole diameter and spacing, eliminating out-of-focus signals, and introducing a frame reduction reconstruction algorithm, this new approach overcomes the limitations of existing techniques in balancing temporal and spatial resolution, thereby greatly expanding the application range of super-resolution imaging within organisms.
With the rapid development of biomedicine, there is an increasing need to reveal the three-dimensional fine structures and capture rapid biological dynamic processes at the tissue level. Confocal laser scanning microscopy (CLSM) has been considered the preferred choice for deep imaging in scattering tissues due to its excellent optical sectioning capability and versatility. However, reducing the pinhole size to enhance resolution leads to a drop in SNR, limiting imaging quality. Image scanning microscopy (ISM) replaces a single-point detector with a detector array, achieving a two-fold resolution enhancement compared to the diffraction limit while maintaining the optical sectioning capability of CLSM through pixel reassignment and deconvolution algorithms. Compared with other super-resolution microscopy, ISM is more resistant to aberrations and scattering issues caused by deep imaging.
This paper proposes the Multi-Confocal Image Scanning Microscopy (MC-ISM) technique, which generates multi-focal illumination through a pinhole array. Unlike traditional ISM, which scans using a single illumination spot, this parallel configuration, coupled with a single-galvo scan similar to an Archimedean spiral, significantly increases the imaging speed. By optimizing pinhole diameter and spacing, applying optical lock-in detection (OLID) to suppress out-of-focus signals, and improving imaging quality, MC-ISM achieves a two-fold enhancement in three-dimensional resolution through pixel reassignment and further deconvolution post-processing. Additionally, by introducing a multi-image deconvolution reconstruction algorithm for frame reduction, MC-ISM can further increase imaging speeds, improving 16 times over multifocal structured illumination microscopy.
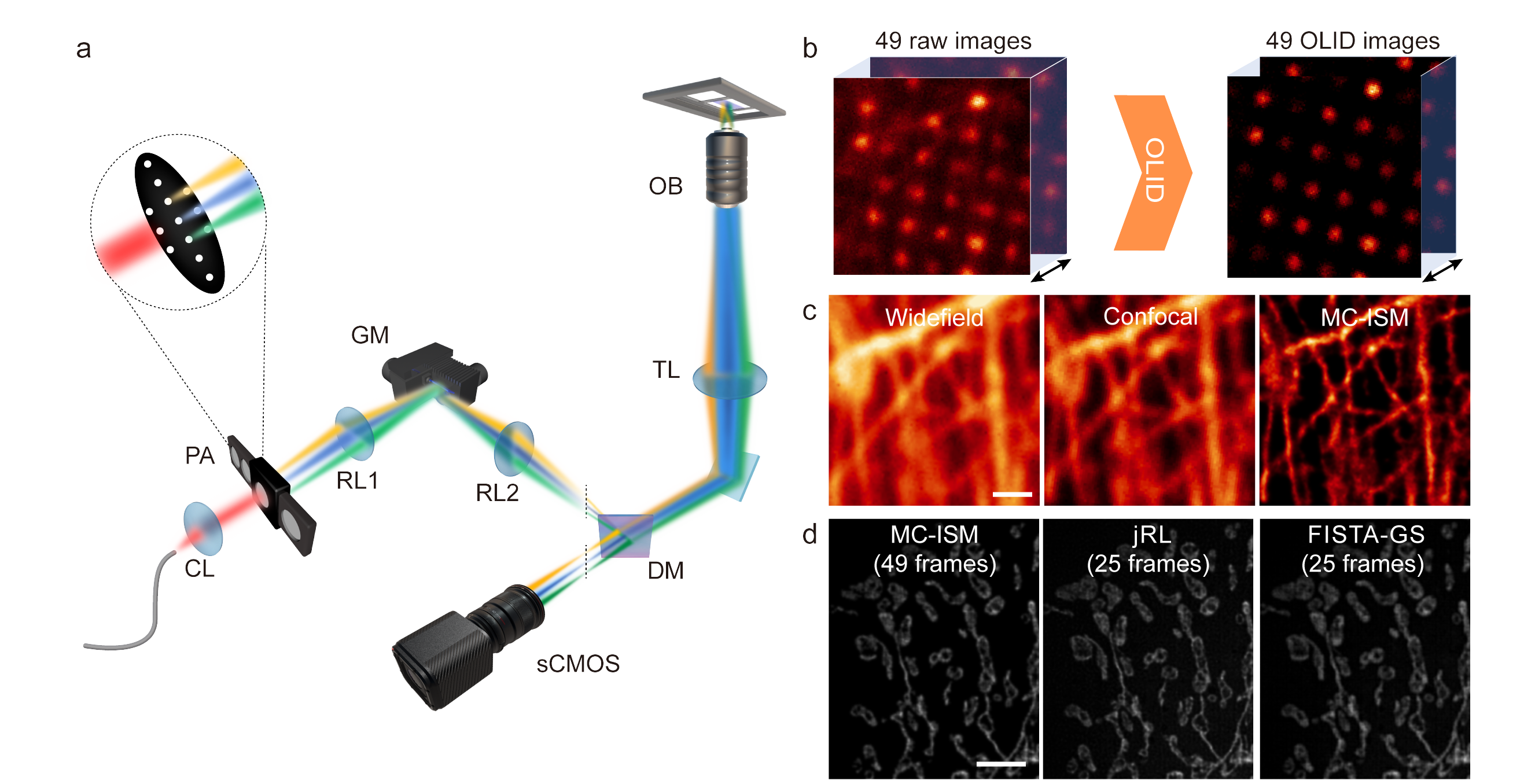
Figure 1 Schematic of the MC-ISM optical path and results of pixel reassignment (PR) and frame reduction reconstruction.
The research team utilized MC-ISM technology to perform three-color 3D super-resolution imaging on sections of mouse kidney. Within a volume of 66.5 μm × 66.5 μm × 12 μm, with an axial interval of 150 nm, filamentous actin, components of the nephron units, and nuclear structures were clearly separated. The lateral and axial resolutions improved to 131 nm and 336 nm, respectively. MC-ISM effectively suppressed out-of-focus signals, demonstrating optical sectioning capabilities comparable to spinning disk confocal microscopy. Furthermore, using a 20× objective with a long working distance, MC-ISM performed deep imaging on DAPI-stained zebrafish heads, also showing exceptional performance, with cell structures clearly visible throughout the imaging volume. Compared with wide-field and confocal modalities, the resolution was significantly enhanced.
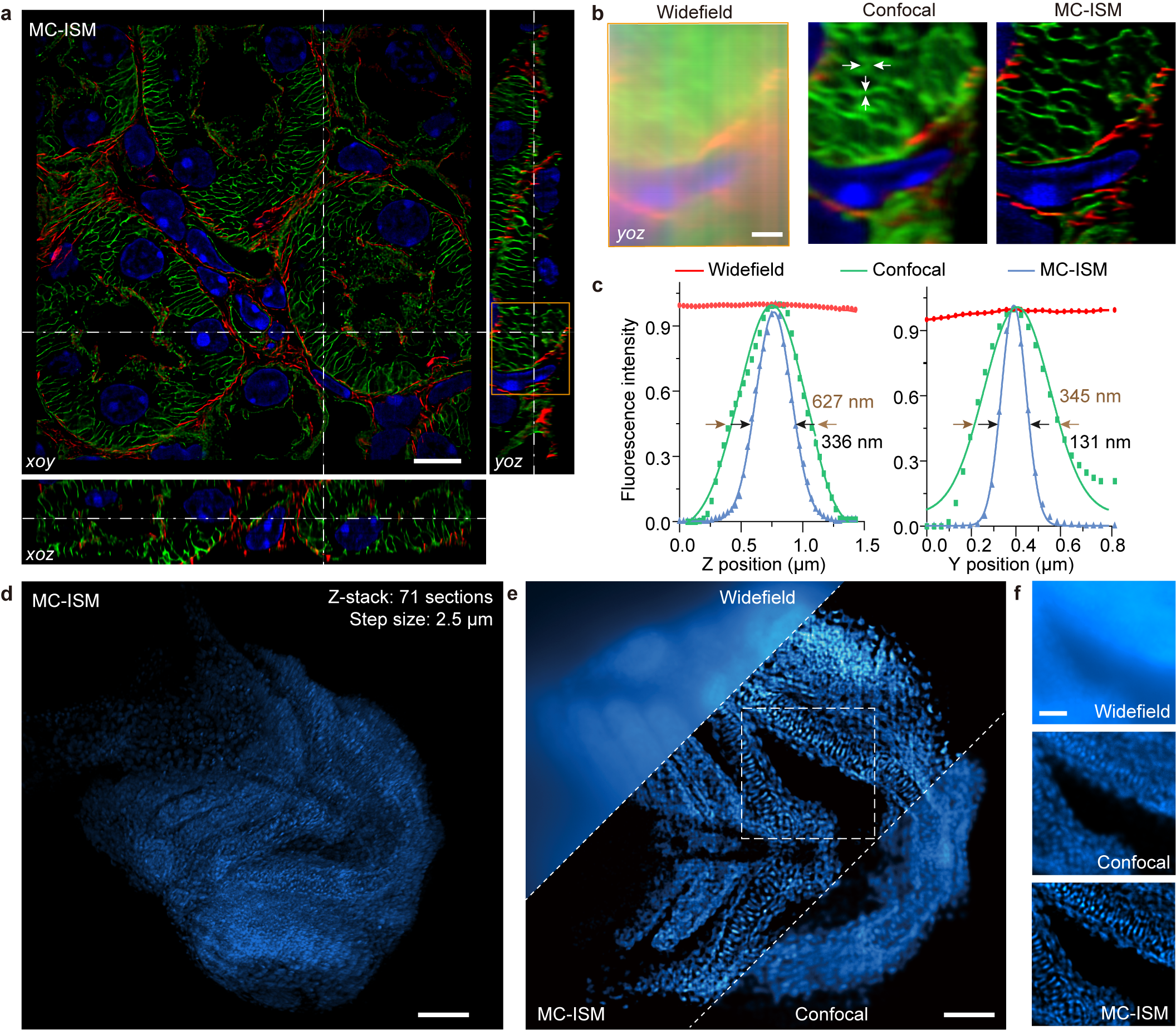
Figure 2 MC-ISM imaging results of mouse kidney tissue sections and zebrafish.
MC-ISM demonstrates enhanced photon collection efficiency due to its simplified detection optical path. Under the same signal-to-noise ratio conditions, the fluorescence intensity of MC-ISM dropped to 67% after 1000 frames of imaging, indicating that its photobleaching is lower than that of OMX structured illumination microscopy, super-resolution spinning disk confocal microscopy, and Airyscan super-resolution microscopy. Additionally, MC-ISM imaged mitochondria in U2OS cells at a speed of 7.5 frames per second, without any swelling of the mitochondria, showing the imaging system's extremely low phototoxicity. In plant cells, MC-ISM successfully overcame tissue scattering and autofluorescence to perform in situ super-resolution imaging of mitochondria in the hypocotyls of Arabidopsis, demonstrating its unique advantage in super-resolution imaging of thick plant tissues. These results prove the superiority and broad application potential of the MC-ISM system in studying mitochondrial dynamics.

Figure 3 MC-ISM imaging results of mitochondrial dynamics in animal cells and Arabidopsis hypocotyl plant tissue.
Breaking the diffraction limit to reveal the true face of cells, using delicate brushstrokes to paint the canvas of life. With specialized hardware design and advanced algorithms working in perfect synergy, MC-ISM delves deep into samples to finely observe deep tissue structures, capturing the ever-changing dynamics of living organisms at high speed. Its low phototoxicity allows cells to naturally display their vitality, thereby revealing the mysteries of complex three-dimensional structures within organisms. The high compatibility of MC-ISM with existing confocal systems, coupled with its cost-effectiveness and ease of operation, makes it a strong contender to lead the next generation of confocal microscopy technology.
The article titled "Expanding super-resolution imaging versatility in organisms with multi-confocal image scanning microscopy" was published in the National Science Review (IF=16.3). The doctoral students Wei Ren and Meiling Guan (now a postdoctoral at the Aerospace Information Research Institute, Chinese Academy of Sciences), and Qianxi Liang are the co-first authors of the article. Professor Peng Xi from the College of Future Technology of Peking University and Teacher Meiqi Li from the School of Life Sciences at Peking University are the co-corresponding authors of this paper. Other significant contributors to the paper include Professor Sodmergen from Peking University, Dr. Boya Jin, doctoral students Guangxing Duan, Liya Zhang, Yiwei Hou, Professor Baoxiang Gao from Hebei University, and master student Xichuan Ge. This work was supported by the National Key R&D Program of China, the National Natural Science Foundation, and also received assistance from Airy Technology Co., Ltd., the National Center for Protein Sciences at Peking University in Beijing, and OptoFem Technology Limited.
Ren, W., Guan, M., Liang, Q., et al. "Expanding super-resolution imaging versatility in organisms with multi-confocal image scanning microscopy." National Science Review (2024): nwae303.
https://academic.oup.com/nsr/advance-article-abstract/doi/10.1093/nsr/nwae303/7742832
1 / 5

