The resolution of optical microscopy is limited by diffraction limit and can only achieve a resolution of ~200 nm. In the past two decades, a series of technologies such as STED, SIM, PALM/STORM, DNA-PAINT, etc., have broken through the limitation of optical diffraction through the modulation of point spread function (PSF), making the optical resolution as low as 20nm, which has been widely used in biology and won the Nobel Prize in Chemistry in 2014.
In 2017, Nobel laureate Stefan Hell (inventor of STED technology) developed MINFLUX technology (MINimal photon FLUXes) and called it "microscopy in the post-Nobel Prize era". MINFLUX further improves the optical resolution to achieve a localization precision of ~1 nm and a spatial resolution of ~5 nm. MINFLUX, as a single-molecule localization technology, combines the characteristics of STED and STORM. Unlike the depletion light of STED, which uses a donut-shaped hollow spot, MINFLUX uses the donut as a excitation light; similar to the principle of GPS positioning, MINFLUX uses a donut beam to excite the fluorescent molecules multiple times, and then the location of the fluorescent molecule can be estimated by statistical methods.
The 1 nm localization precision is already very close to the size of a single molecule. However, the localization precision is not equal to the optical resolution, the resolution has not reached the scale of ~ 1 nm, and there is still room for improvement. Are there new methods to further improve the resolution of MINFLUX?
The Peng Xi laboratory in College of Future Technology has theoretically proved that using two-photon excitation (TPE) in MINFLUX technology can further increase both the localization precision and resolution by 2 times, and obtain the precision of less than 1 nm. The conclusion is titled “ Two-photon MINFLUX with doubled localization precision ” and published in the latest issue of eLight . The results of the article show that the two-photon MINFLUX (Two-photon MINFLUX, 2p-MINFLUX) can not only improve the localization precision by 2 times, but also have a more direct multi-color imaging potential.
This work has been highlighted by the research group of Professor Fernando Stefani, an authoritative super-resolution expert, and published in Light: Science & Applications with the title of “ Multiphoton single-molecule localization by sequential excitation with light minima ”[https://doi.org/10.1038/s41377-022-00763-2]. Comments point out that this work provides a prospective theoretical basis for both the introduction of multiphoton effects into MINFLUX technology, and the future application of MINFLUX to deep biological imaging and achieving higher localization precision.
The localization precision of MINFLUX is represented by the Cramér–Rao Bound lower bound (CRB). Through mathematical derivation, the article rigorously proves that the two-photon effect makes the CRB changed to 1/2 of the original, that is, the localization precision is increased by 2 times, and the improvement is true for the three dimensions of xyz. At the same time, the authors and others further analyzed the influence of various experimental parameters on the localization accuracy, including the number of fluorescent photons, bias length, and signal-to-background ratio. Same as the existing MINFLUX technology (1p-MINFLUX), in 2p-MINFLUX, the signal-to-background ratio determines the minimum value of the bias length, which ultimately limits the achievable localization accuracy. In fact, one of the characteristics of two-photon technology is to reduce the fluorescence background out of focus. Since two-photon excitation only occurs at the focal point and no excitation elsewhere in three-dimensional space, two-photon MINFLUX has a lower background than single-photon MINFLUX, which may further improve the localization accuracy. In order to directly compare the performance of the two-photon MINFLUX and the existing MINFLUX technology, the numerical selection of each parameter is consistent in the simulation.
Figure 1 visually compares and demonstrates the imaging process and results of 2p-MINFLUX and existing 1p-MINFLUX. On the one hand, under the condition that 2p-MINFLUX and 1p-MINFLUX collect the same number of fluorescent photons, structures that cannot be resolved by 1p-MINFLUX can be resolved by 2p-MINFLUX (Fig. 1b,c). On the other hand, when 2p-MINFLUX and 1p-MINFLUX achieve the same localization accuracy, 2p-MINFLUX only needs 1/4 of photons that of 1p-MINFLUX (Fig. 1c,d).
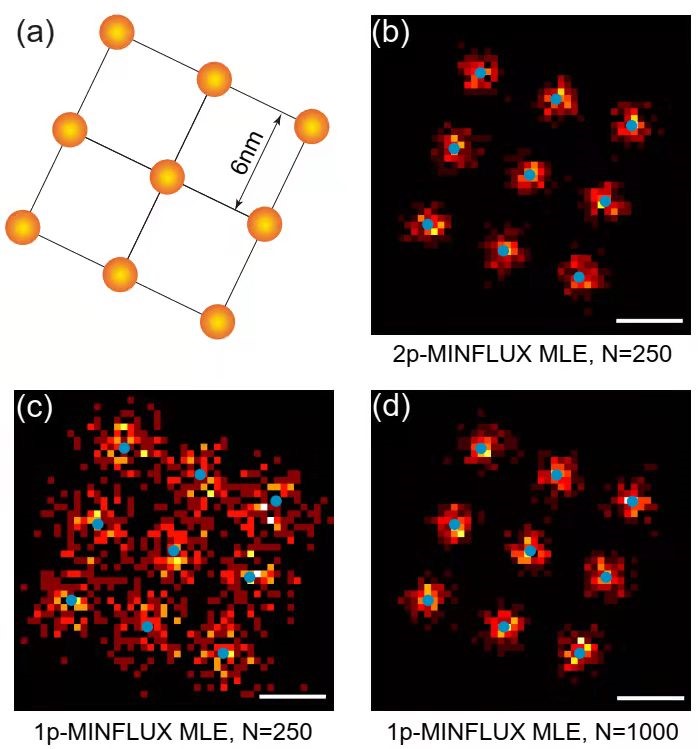
Figure 1. Simulated imaging results of two-photon MINFLUX. This result intuitively reflects the relationship between "2 times the localization precision" and "1/4 times the number of required photons" in 2p-MINFLUX.
In summary, the resolution of MINFLUX technique can be promoted by the two-photon nonlinear effect, which can improve both the localization precision and resolution by 2 times, or reduce the required number of fluorescent photons by 4 times. The resolution of fluorescence microscopy can be further approached to the limit of the scale of individual molecules. The authors conclude that, due to the existence of two-photon nonlinear effects, 2p-MINFLUX may have more direct multi-color imaging capabilities; at the same time, 2p-MINFLUX can be utilized not only as a super-resolution imaging method, but also as a high-speed multi-color single molecule method for tracking. This technology can further promote basic research in the field of biomedicine, especially the research on biological interactions such as nucleic acid-protein interactions and virus-cell interactions.
The first author of this work is Zhao Kun, a Ph.D. student in the College of Future Technology of Peking University, who is also a student in the Peking University-Georgia Institute of Technology-Emory University joint Ph.D. program. The collaborators are Xinzhu Xu (also joint Ph.D.) and Wei Ren from the College of Future Technology of Peking University, and Professor Dayong Jin from Southern University of Science and Technology. Professor Peng Xi is the corresponding author of this work. This work was supported by the Beijing Municipal Science and Technology Commission, the Shenzhen Municipal Science and Technology Commission, the National Natural Science Foundation of China, and the Ministry of Science and Technology.
Link: https://elight.springeropen.com/articles/10.1186/s43593-021-00011-x

