The Daoist concept "From Taiji arises Liangyi, and from Liangyi emerge Sixiang" mirrors cell fate determination in embryonic development: from a single zygote, sequential divisions through the two-cell and four-cell stages culminate in lineage-specific developmental blueprints. This biological 'Taiji differentiation' is orchestrated by epigenetic reprogramming, which precisely governs zygotic genome activation (ZGA), totipotency maintenance and loss, and the first cell fate determination. Previous studies using low-input ChIP-seq have revealed extensive chromatin state remodeling—particularly histone modification reprogramming—during preimplantation mammalian embryogenesis[1-6]. However, whether it is possible to transcend the limitations of traditional genetic lineage tracing and single-cell transcriptomics by establishing a single-cell, time-resolved, genome-wide, and multimodality histone modification informative approach to decipher embryonic development and underlying key epigenomic drivers remains an open question.
On February 26, 2025, a team led by Dr. Aibin He from Peking University published a groundbreaking study in Nature titled "Genome-coverage single-cell histone modifications for embryo lineage tracing". The study introduces two novel techniques, TACIT and CoTACIT, which enables genome-wide profiling of histone modifications at single-cell resolution. This work establishes the first spatiotemporally resolved multi-dimensional histone modification atlas of mouse pre-implantation embryos and reconstructed an epigenetic lineage tree. The research elucidates the epigenomic control mechanisms at single-cell resolution from zygotes to blastocysts, pointing characteristic histone modifications and regulatory elements defining totipotency. Additionally, it identifies key transcription factors and transposable elements governing totipotency exit and first cell fate priming. These findings provide a panoramic epigenetic perspective on molecular and cellular regulation during early embryogenesis.

Breaking Techical Bottlenecks: TACIT and CoTACIT Enable Panoramic Single-Cell Histone Modification Profiling
Conventional single-cell histone modification assays are hindered by high input requirements and limited resolution, making them difficult to apply to low-input early embryo samples. To overcome this, Aibin He's team developed TACIT (Target Chromatin Indexing and Tagmentation), which incorporates a series of optimizations, including methanol fixation, enhanced Protein A-Tn5 transposase activity, and single-tube reactions to prevent cell and DNA loss. These improvements increased the valid reads per cell by nearly 50-fold, with a median of 492,556 reads per early embryo cell. TACIT operates robustly with as few as 20 cells and demonstrates superior signal-to-noise ratios over existing methods (including the team’s previous itChIP-seq and CoBATCH, as well as Cut&Tag-like techniques). The team further developed CoTACIT (Combined assay of Target Chromatin Indexed and Tagmented), enabling simultaneous detection of multiple histone modifications within the same cell.
Using TACIT and CoTACIT, the researchers generated a genome-wide dynamic histone modification landscape across 3,749 single cells from mouse pre-implantation embryos, covering six histone modifications (H3K4me1, H3K4me3, H3K27ac, H3K36me3, H3K27me3, and H3K9me3) and one histone variant (H2A.Z). This dataset captures nearly all functional regulatory elements, including promoters, enhancers, gene bodies, heterochromatin, and histone variant-enriched regions. By integrating multi-modal histone modification data with scRNA-seq data as a bridge, the team reconstructed the chromatin state dynamics of early embryos using a ChromHMM-based framework[7-9].
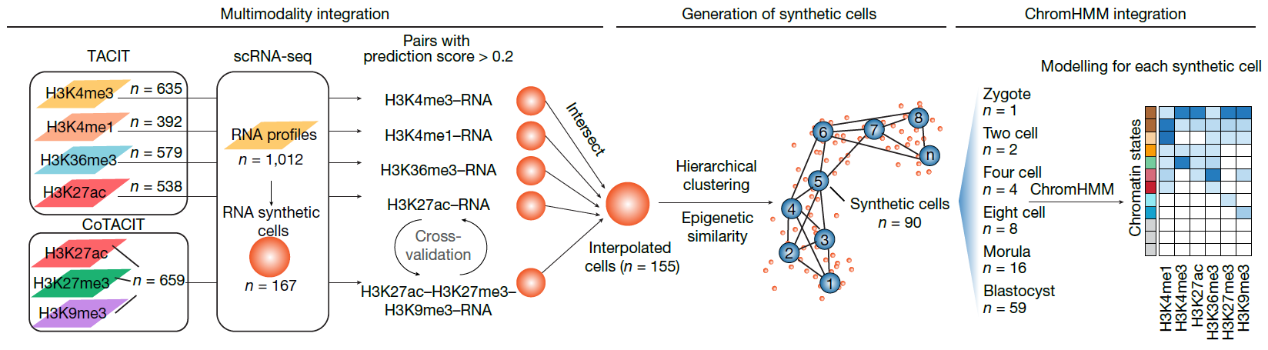
Fig 1. Schematic of the multimodality integration analysis pipeline.
Epigenetic Heterogeneity at the Two-Cell Stage: Early Clues to Fate Determination
Just as no two leaves are identical, cellular identity divergence begins even in morphologically homogeneous two-cell embryos. The research team discovered that active histone modifications such as H3K27ac already exhibit significant inter-embryo heterogeneity in two-cell embryos. Using embryo-barcoded TACIT to track epigenomic profiles of sibling blastomeres with the same embryo, the team demonstrated that approximately 31%-45% of two-cell embryos display intra-embryo epigenetic differences. This heterogeneity closely correlates with ZGA progression. Multi-modal analyses further revealed that zygotes and low-ZGA two cell blastomeres (2cell_1) contain a multivalent chromatin state (co-marked by six histone modifications), whereas high-ZGA two-cell counterparts (2cell_2) do not exhibit this state.
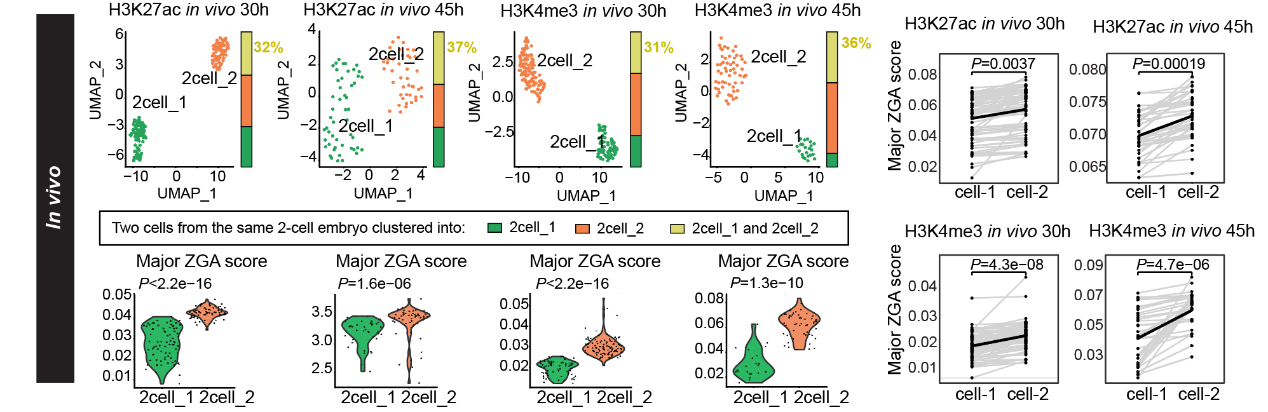
Fig 2. Validation of 2cell stage heterogeneity with embryo-barcoded TACIT
Epigenetic Lineage Prediction: Totipotency Exit and First Cell Fate Pre-Determination
To further explore the epigenetic contributions to cell fate, the researchers leveraged multimodal chromatin state information to define cell identities. Machine learning identified 2,583 totipotency-associated genomic regions, with 31% overlapping with known totipotency genes and 41% enriched with transposable elements such as MERVL. They then analyzed transcription factor motifs enriched in these totipotency-associated genomic regions and confirmed, through CRISPRa-based functional validation experiments, that newly identified transcription factors such as CEBPG, LBX1, and ESR1 could reprogram embryonic stem cells toward a totipotent-like state.
Additionally, the team identified epigenomic regulatory regions associated with ICM (inner cell mass) and TE (trophectoderm) specialization, enabling accurate prediction lineage propensity before the blastocyst stage. siRNA experiments validated that previously unreported transcription factors—YY2, CEBPB, SMAD2, and HNF4A—regulate ICM identity, whereas KLF6 and HIF1A drive TE differentiation. Interestingly, they found that early embryos exhibit distinct lineage tendencies as early as the four-cell stage.
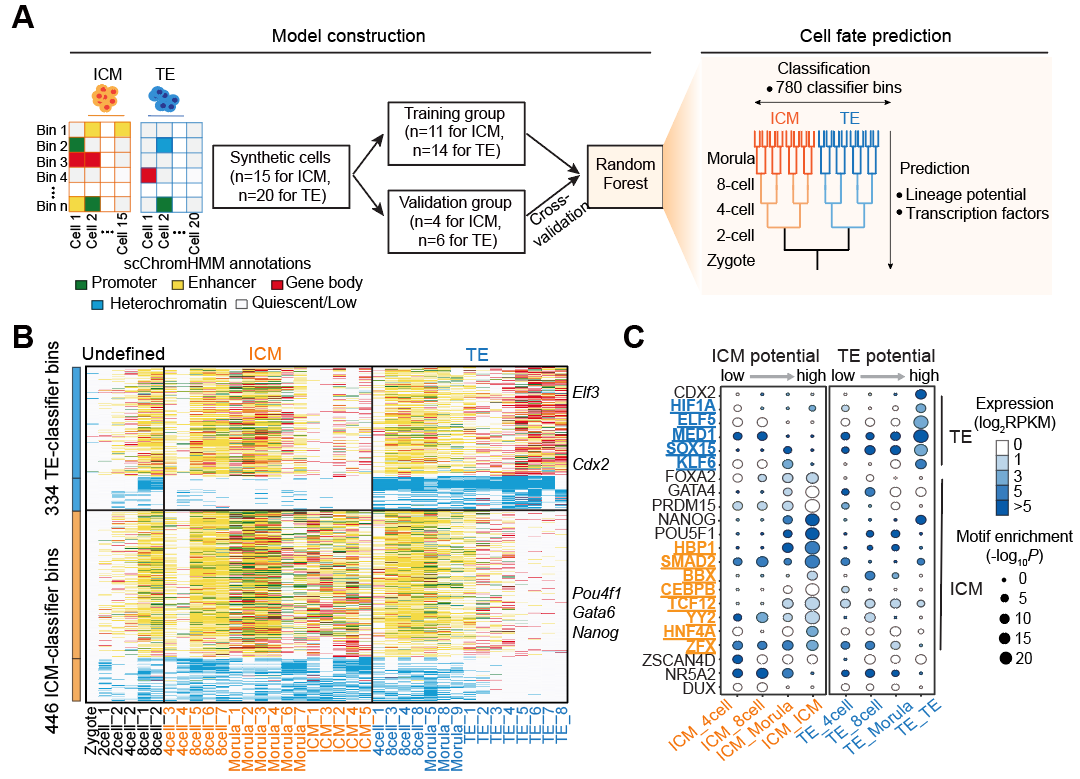
Fig 3. Lineage-Associated Regulatory Elements and Transcription Factors. | A. A computational pipeline for constructing a random forest training model to identify classifier bins associated with lineage specification. B. Heatmap displaying chromatin-state annotations in all 780 classifier bins. C. TF motifs identified during ICM or TE lineage specification.
Technological Innovations and Future Perspectives
This study has mapped a time-resolved single-cell epigenomic lineage tree (Fig. 4) spanning six histone modifications across mouse embryogenesis from zygote to blastocyst. It revealed the epigenetic mechanisms underlying cellular heterogeneity and first fate pre-determination within embryos, establishing a new paradigm for studying embryonic development and cell fate regulation. Beyond providing fresh insights into early embryonic development, the research methodology can also be extended to human diseases, such as decoding the epigenetic basis of tumor heterogeneity, and regenerative medicine.
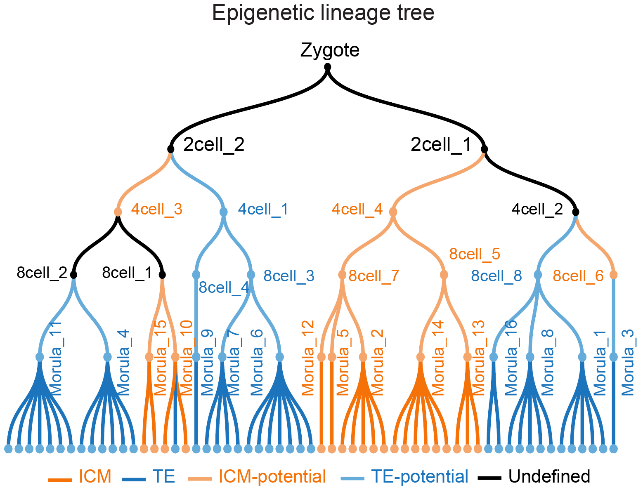
Fig4. Epigenetic lineage tree based on chromatin state annotations of synthetic cells.
Original Article Link: https://doi.org/10.1038/s41586-025-08656-1
REFERENCES:
1 Xu, R., Li, C., Liu, X. & Gao, S. Insights into epigenetic patterns in mammalian early embryos. Protein Cell 12, 7-28, doi:10.1007/s13238-020-00757-z (2021).
2 Xia, W. & Xie, W. Rebooting the Epigenomes during Mammalian Early Embryogenesis. Stem Cell Reports 15, 1158-1175, doi:10.1016/j.stemcr.2020.09.005 (2020).
3 Zernicka-Goetz, M., Morris, S. A. & Bruce, A. W. Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat Rev Genet 10, 467-477, doi:10.1038/nrg2564 (2009).
4 Dahl, J. A. et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 537, 548-552, doi:10.1038/nature19360 (2016).
5 Liu, X. et al. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 537, 558-562, doi:10.1038/nature19362 (2016).
6 Zhang, B. et al. Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature 537, 553-557, doi:10.1038/nature19361 (2016).
7 Ernst, J. & Kellis, M. ChromHMM: automating chromatin-state discovery and characterization. Nat Methods 9, 215-216, doi:10.1038/nmeth.1906 (2012).
8 Stuart, T. et al. Comprehensive Integration of Single-Cell Data. Cell 177, 1888-1902 e1821, doi:10.1016/j.cell.2019.05.031 (2019).
9 Zhang, B. et al. Characterizing cellular heterogeneity in chromatin state with scCUT&Tag-pro. Nat Biotechnol 40, 1220-1230, doi:10.1038/s41587-022-01250-0 (2022).

