Yange Niu,Rui Liu,Chengcheng Guan,Yuan Zhang,Zhixing Chen,Stefan Hoerer,Herbert Nar &Lei Chen✉
Abstract
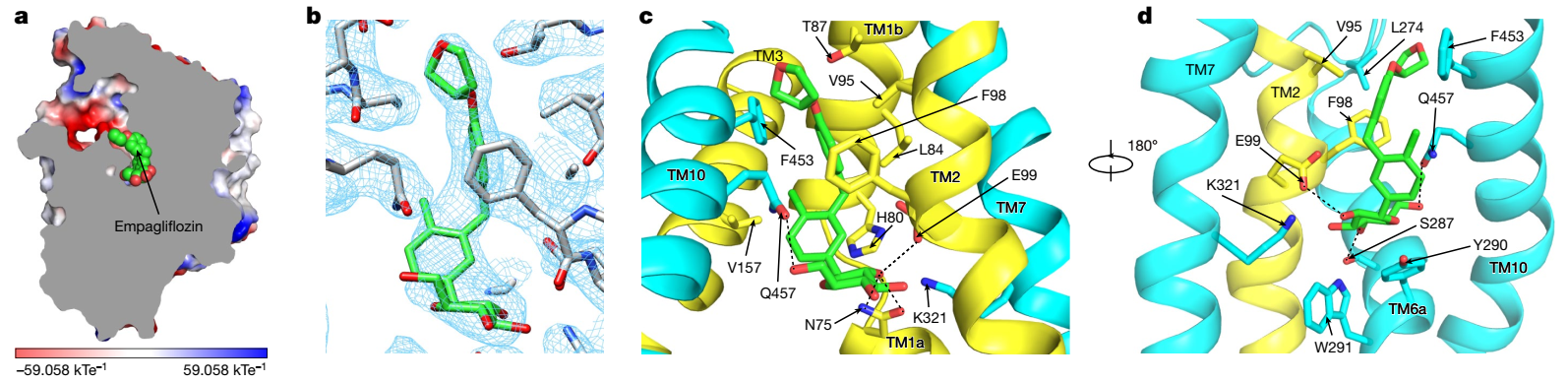
Human sodium–glucose cotransporter 2 (hSGLT2) mediates the reabsorption of the majority of filtrated glucose in the kidney1. Pharmacological inhibition of hSGLT2 by oral small-molecule inhibitors, such as empagliflozin, leads to enhanced excretion of glucose and is widely used in the clinic to manage blood glucose levels for the treatment of type 2 diabetes1. Here we determined the cryogenic electron microscopy structure of the hSGLT2–MAP17 complex in the empagliflozin-bound state to an overall resolution of 2.95 Å. Our structure shows eukaryotic SGLT-specific structural features. MAP17 interacts with transmembrane helix 13 of hSGLT2. Empagliflozin occupies both the sugar-substrate-binding site and the external vestibule to lock hSGLT2 in an outward-open conformation, thus inhibiting the transport cycle. Our work provides a framework for understanding the mechanism of SLC5A family glucose transporters and also develops a foundation for the future rational design and optimization of new inhibitors targeting these transporters.